The article presents the basic concepts of non-atopic eosinophilic asthma separately and endotypes and phenotypes of asthma in children and adults;
Until recently, bronchial asthma (AD) in children and adults was defined as allergic (or atopic) and non-allergic (or non-atopic). The main difference between these two types of AD is based on the presence/absence of clinical symptoms of an allergic reaction and sensitization according to allergic diagnostics (skin tests and determination of specific IgE (sIgE) antibodies to various allergens).
However, thanks to the development and implementation of methods for sampling from the respiratory tract (induced sputum, cytology of nasal mucus), new analytical approaches for biological samples (evaluation of the microbiome of the respiratory tract and epigenetics), and new biostatistical methods, scientists managed to go beyond the old classification of atopic / non-atopic AD and eosinophilic asthma / non-eosinophilic AD [1, 2].
Are we known today about eosinophilic asthma?
It would seem that a detailed history of the disease, including concomitant (comorbid) diseases, a study of the function of external respiration with a bronchodilator test, a general blood test, the detection of IgE antibodies, and counting peripheral blood eosinophils is information that is enough for the doctor to diagnose at the first stage patient allergic or non-allergic asthma.
The clinical symptoms for both types of the disease are the same.
(wheezing, shortness of breath, the sensation of pressure in the chest, and cough), which vary in time and intensity of manifestations and reversibility of bronchial obstruction. However, even ten years ago, scientists began to distinguish various subgroups of patients with AD based on the immunological features of the course of the disease, biomarker data, response to specific pharmacotherapy, and long-term prognosis. These are the so-called phenotypes – unique clinical characteristics or subtypes of AD.
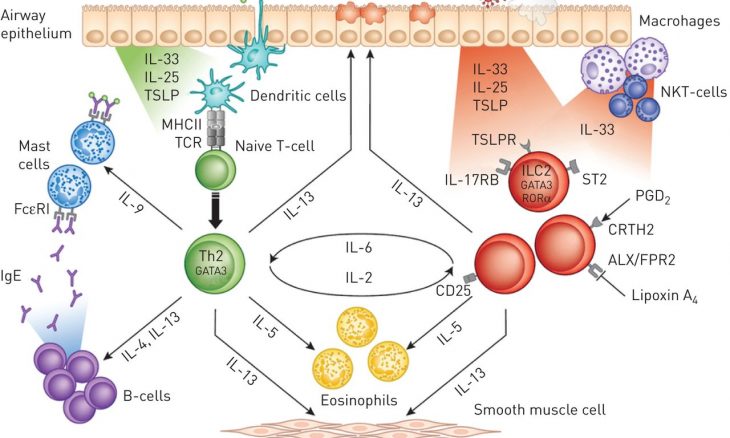
The study of pathophysiological processes in various asthma variants (phenotypes) gave rise to the concept of “endotypes” – “an asthma subtype characterized by a specific functional or pathophysiological mechanism” of development. Indeed, the results of cluster analysis revealed several heterogeneous asthmatic subgroups with different pathophysiology and a different response to treatment.
Subgroups were classified into “endotypes” based on various traits, including sIgE levels, the number of eosinophils in induced sputum, and fractional expired nitrogen oxide (FeNO) characterized by a specific functional or pathophysiological mechanism of development.”
Indeed, the results of cluster analysis revealed several heterogeneous asthmatic subgroups with different pathophysiology and different responses to treatment.
Subgroups were classified into “endotypes” based on various traits, including sIgE levels, the number of eosinophils in induced sputum, and fractional expired nitrogen oxide (FeNO). They were characterized by a specific functional or pathophysiological mechanism of “development.” Indeed, the results of cluster analysis revealed several heterogeneous asthmatic subgroups with different pathophysiology and a different response to treatment. Subgroups were classified into “endotypes” based on various traits, including sIgE levels, the number of eosinophils in induced sputum, and fractional expired nitrogen oxide (FeNO) [3–8].
As further studies have shown, “Th2-type inflammation in asthma is present in most, but absent in many” [11]. In other words, it became clear that not one, but several endotypes can participate in the formation of a non-atopic BA.
Distinguish the Th2-endotype of AD, which includes allergic asthma – closely associated with atopy,
sIgE production and eosinophilic inflammation. Several subgenotypes may exist within this endotype (with high levels of IL-5, high levels of IL-13, or high levels of total IgE) [9].
The non-Th2 endotype is typical for AD patients who do not have atopy and allergy symptoms; that is, this endotype determines non-atopic asthma [1, 10, 11]. At the same time, this is also a heterogeneous group, which is associated more with neutrophilic inflammation in the airways and such cytokines like IL-17, IL-1b, TNF-α [22], and the chemokine receptor (CXCR2) [12, 13].
Neutrophilic Endotype
Neutrophilic inflammation is associated with the development of bronchial hypersensitivity and airway remodeling, especially in patients with non-atopic AD. This type of inflammation with a predominance of Th1 / neutrophils is accompanied by a decrease in sensitivity to steroids, and neutralization of TNF-α restores it [14]. Obviously, with this endotype, the atopic component of inflammation is also unlikely.
Mixed Endotype Th2 / Th17
Such an inflammation mechanism has been described relatively recently in AD and implies the differentiation of Th2 cells into double-positive Th2 / Th17 cells [15]. This mixed endotype has been little studied.
Thus, as can be seen from the above, most AD endotypes correspond to non-atopic asthma.
As for AD phenotypes, previously accepted criteria (severe, mild, therapy-resistant asthma, etc.) also underwent substantial changes [17].
An attempt to unravel the asthma-allergic associations
It is known that almost 50% of children experience shortness of breath in the first year of life, although only 20% will have symptoms of shortness of breath in later childhood [18, 19]. In some children, the “wheezing” phenotype (wheezing, wheezing) continues until late childhood, while in others, adolescence and adulthood.
In 2008, PRACTALL experts proposed the following AD phenotypes in children: virus-induced; allergen-induced; unresolved asthma; asthma of physical stress. Only in this document do scientists refer to persistent AD in children without appropriate allergic sensitization as “unresolved asthma”.
It is important to note at the same time that, according to the analysis of bronchial biopsy. Samples obtained in children with wheezing (average age five years; range 2–10 years), pathomorphological changes (thickened basement membrane, increased number of eosinophils, and cytokine expression) did not differ in children with non-atopic and atopic wheezing.
Phenotypes can change over time due to differences in the severity of symptoms and risk factors. Undoubtedly, therapeutic intervention can also change the course of the disease over time. According to A. Boudier et al., after 10 years of monitoring adult patients with AD (n = 3,320). The phenotype persisted in 78% of the study participants.
Treatment
The treatment of patients with non-atopic asthma does not differ from the treatment of allergic asthma. It includes inhaled corticosteroids with the addition of long-acting β-agonists. To achieve disease control, additional therapy consists of increasing the dose of ICS, adding anti leukotriene drugs, or theophylline [36]. The response to drugs can vary significantly because it is not clear whether AD includes a combination of different conditions, or is it a single state with several mechanisms and phenotypes [37]. The heterogeneity of phenotypes and the different response to anti-asthma drugs, especially in young children and patients with severe AD. Confirm the importance of personalizing therapy in each case. So, Fitzpatrick et al., in a recent study, showed that in preschool children with persistent AD.
A weak short-term response to the treatment of inhaled corticosteroids in patients. Non-atopic asthma associate with the fact that these drugs primarily intend to suppress eosinophilic inflammation.
Patients with severe asthma with a Th1 endotype and neutrophilia in induced sputum may benefit from macrolide therapy. In particular, a recent study once again showed that azithromycin reduces asthma exacerbations—both severe eosinophilic and non-eosinophilic asthma, which indicates the immunomodulating effect of macrolides.
Recently, omalizumab is also prescribed for non-atopic asthma, since in such patients, the level of total IgE is often increased, including at the level of bronchial tissue [41]. Recent advances in the treatment of patients with non-atopic asthma, but with clear signs of high Th2 response, relate to drugs such as mepolizumab or reslizumab that block IL-5 [42, 43].
Approaches to the treatment of various asthma endotypes
Clinical observation
Patient I., 14 years old, consulted an allergist with an unspecified diagnosis: “Chronic bronchitis of unknown etiology.”
Anamnesis of life. The heredity of allergic and other chronic diseases of the respiratory tract burden. The mother had atopic dermatitis in her childhood; there were no further complaints. A girl from the first physiological pregnancy, the first independent birth. Apgar score at birth 8/9 points. Of the diseases – rare acute respiratory infections, chickenpox.
Medical history.
For the first time, coughing without wheezing appeared at the age of 8 years. The cough associate with any provoking factors (contact with allergens, cold, physical activity). From the age of 13, nasal congestion appeared. With the preservation of smell and without other symptoms of allergic rhinitis (itchy nose, runny nose, sneezing).
according to x-ray and CT of the lungs – there is no data for chronic pathology; Ultrasound of the abdominal organs – without pathology; results of an allergological examination (skin tests, determination of sIgE for inhaled allergens – were negative); there are no data for gastroesophageal reflux. In a general blood test – without pathological changes.
On examination, changes from the internal organs detect.
When examining the function of external respiration in a child, all “curve-flow” indicators are standard. However, the test with bronchodilator salbutamol 200 mcg is sharply positive (FEV 1 + 22%).
After two months, according to the mother, the child has coughing attacks less frequently, although they have not entirely stopped. Communication with triggers is also missing.

